Atomic table periodic structures structure basics lewis dot elements science 8th grade vhmsscience weebly completed end pages
Table of Contents
Table of Contents
Are you struggling with drawing the atomic structure of elements? Have you been searching for a comprehensive guide that makes the process easier? Look no further! In this post, we will share with you the steps and tips on how to draw atomic structure of elements.
Many students find drawing the atomic structure of elements as a challenge. Whether you are a beginner or have tried drawing the structure before, you may find it daunting to keep track of the number of protons, electrons, and neutrons in an atom. Moreover, understanding the different energy levels of electrons and how to represent them can be confusing.
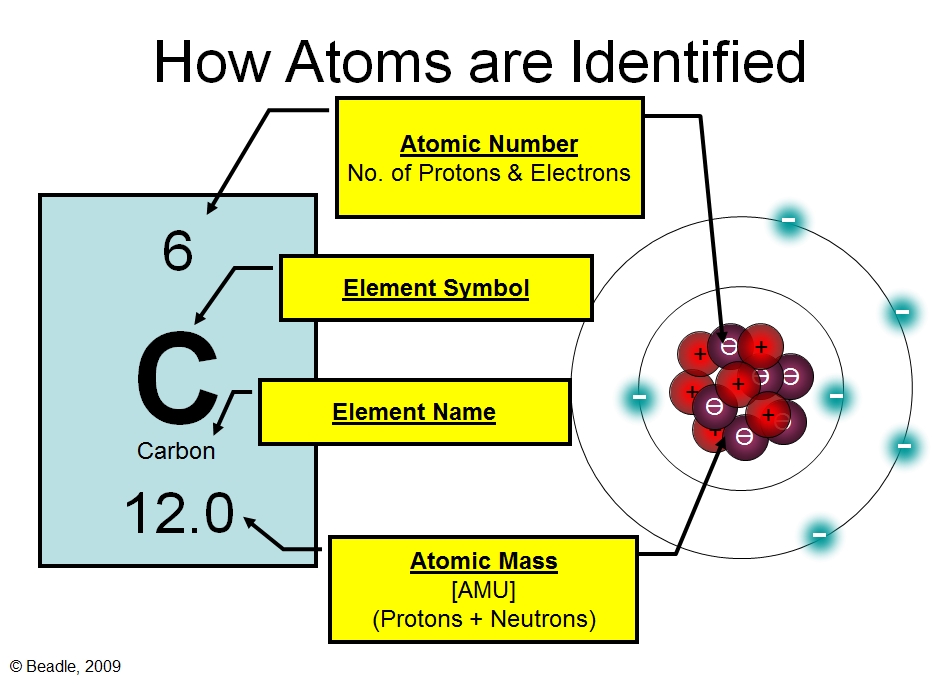
The first step in drawing the atomic structure of an element is identifying the atomic number and the number of electrons. The atomic number represents the number of protons in an atom. To determine the number of electrons, refer to the position of the element in the periodic table. Next, draw the nucleus with the number of protons and neutrons. Then, draw the electrons in their respective energy levels or shells, the first shell containing two electrons and the second shell containing eight electrons.
In summary, drawing the atomic structure of elements requires identifying the number of protons and electrons, drawing the nucleus with the protons and neutrons, and representing the electrons in their respective energy levels.
How to Draw Atomic Structure of Elements Step-by-Step
When I was in high school, I struggled with drawing atomic structures in Chemistry class. However, with practice and guidance, I was able to master the process. Here are the steps and tips on how to draw atomic structure of elements:
 Tips and Tricks on How to Draw Atomic Structure of Elements
Tips and Tricks on How to Draw Atomic Structure of Elements
It’s normal to make mistakes when drawing the atomic structure of elements. Here are some tips and tricks to help you master the process:
 ### Understanding the Different Energy Levels of Electrons
### Understanding the Different Energy Levels of Electrons
Electrons occupy different energy levels or shells around the nucleus. The first shell can accommodate two electrons, while the second shell can hold up to eight electrons. It’s important to understand the electron configuration of an element before drawing its atomic structure. For example, oxygen has six electrons in its valence shell, so its atomic structure would have two electrons in the first shell and four electrons in the second shell.
Representing Electrons in Energy Levels or Shells
Representing the electrons in their respective energy levels can be tricky. It’s important to follow certain rules, such as the aufbau principle, which states that electrons fill the lowest energy orbitals first. Additionally, remember to draw the electrons with arrows to show their spin orientation.
Frequently Asked Questions about How to Draw Atomic Structure of Elements
Q: What is the atomic number?
The atomic number represents the number of protons in an atom. It’s usually denoted by the letter Z in chemistry.
Q: What is the difference between the atomic number and mass number?
The atomic number represents the number of protons in an atom, while the mass number represents the total number of protons and neutrons.
Q: What are valence electrons?
Valence electrons are the electrons in the outermost shell of an atom. They are responsible for the chemical properties of an element, such as its reactivity.
Q: What is the aufbau principle?
The aufbau principle states that electrons fill the lowest energy orbitals first. This means that electrons occupy the energy levels closest to the nucleus first before moving to higher energy levels.
Conclusion of How to Draw Atomic Structure of Elements
As demonstrated, drawing the atomic structure of elements requires identifying the atomic number, the number of electrons, and representing the electrons in their respective energy levels. With patience and practice, mastering this process will become easier. Remember to follow the tips and tricks shared in this post and consult resources such as the periodic table to aid in the process.
Gallery
Drawing Atoms (NCEA L1 & Junior Science) - YouTube
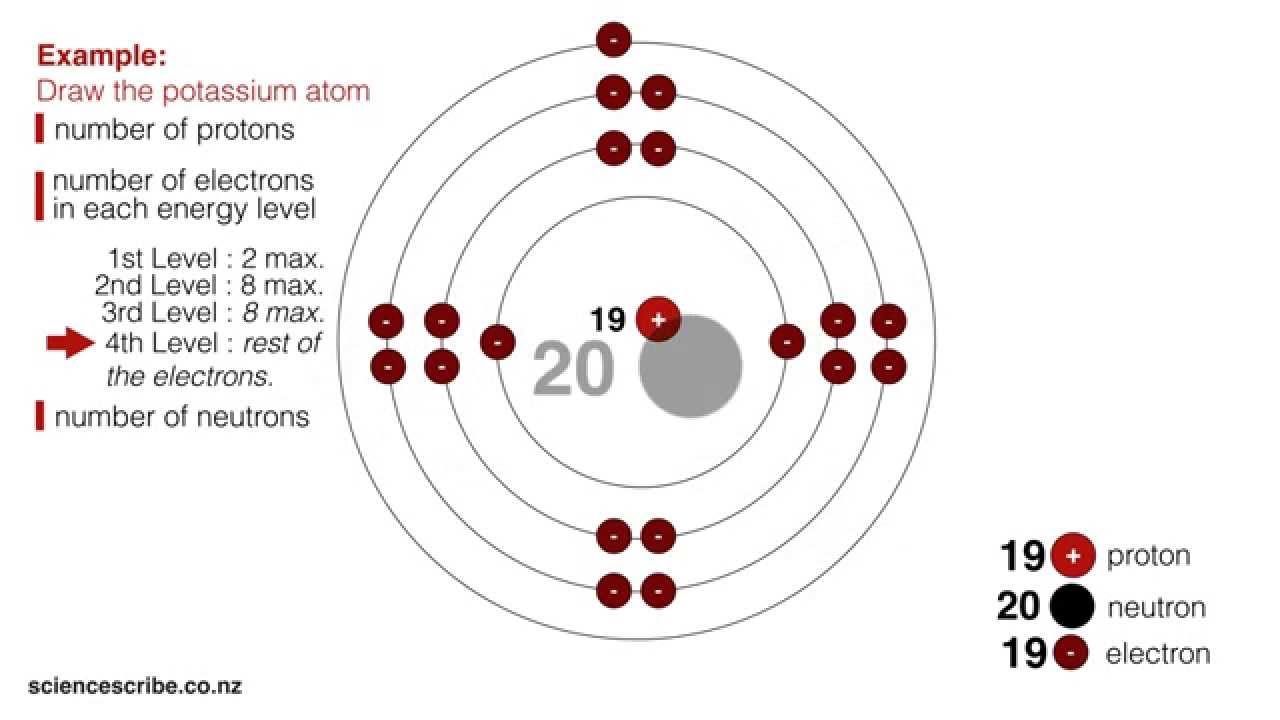
Photo Credit by: bing.com / drawing atoms science ncea
Structure Of An Atom (Learn) : Chemistry : Class 9 : Amrita Vidyalayam

Photo Credit by: bing.com / structure elements atomic schematic eighteen atom diagram class chemistry fig
Atomic Structures & The Periodic Table - VISTA HEIGHTS 8TH GRADE SCIENCE

Photo Credit by: bing.com / atomic table periodic structures structure basics lewis dot elements science 8th grade vhmsscience weebly completed end pages
About - Atomic Structure

Photo Credit by: bing.com / helium atom atoms atomic draw model drawing number shell project structure electrons element mass outer sodium diagram does example each
March 24th, 2015 - JCScience.ie
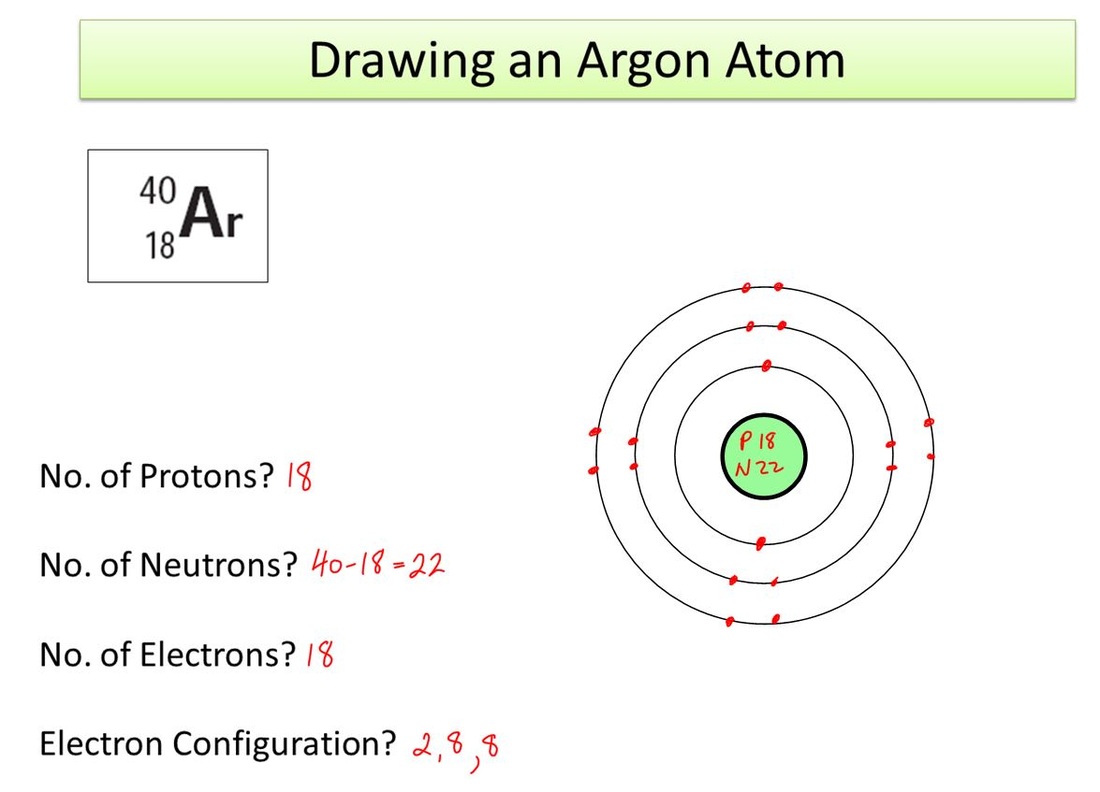
Photo Credit by: bing.com / atoms 24th